Welcome
Platelets, Von Willebrand Factor and Complement
Joel Moake, M.D., and his Laboratory in Hemostasis-Thrombosis Research have had long-term collaborations with engineers at Rice University. In 1975, Dr. Moake began conducting research with Professor J. David Hellums, who served as chair of the Department of Chemical Engineering (1970-1976) and dean of the George R. Brown School of Engineering (1980-1988), and Professor Larry V. McIntire, who established the J. W. Cox Laboratory for Biomedical Engineering, served as chair of the Department of Chemical Engineering (1981-1989), and was founding chair of the Department of Bioengineering (1997). In recent years, the Moake Laboratory in Hematological Research has worked collaboratively with Rice professor and biophysicist Ching-Hwa Kiang and bioengineering professor Jane Grande-Allen.
Investigations in the Moake Laboratory have shown that flow conditions play an important role in determining human blood platelet reactions and that the fluid shear stresses associated with flow in blood vessels can lead to stimulation or functional alterations of platelets. The group has determined the extent of platelet responses to various agonists, and altered the structure and function of von Willebrand factor (VWF) – a multimer important in hemostasis and thrombosis. Studies in carefully controlled, defined shear fields have been especially significant in elucidating the mechanics and kinetics of platelet-VWF adhesive interactions. The instruments developed for these shear studies have included rotational viscometers and parallel plate flow chambers. More recently, the laboratory has utilized immunofluorescent microscopy to elucidate the molecular linkage between VWF and the alternative complement pathway. These latter studies have involved collaborators from institutes of Texas Medical Center, Texas Children’s Hospital and Baylor College of Medicine.
Projects in the Moake laboratory are innovative and medically relevant to the pathophysiology and treatment of thrombotic microangiopathies, arterial thrombosis, and immune inflammatory diseases. One therapeutic agent, N-acetylcysteine, an inexpensive drug previously approved by the FDA for other conditions, has recently been found by the laboratory to have efficacy in humans as an anti-arterial thrombotic agent. Another molecule, heat-inactivated complement factor B, has been found to block the alternative complement pathway and to be a potential anti-inflammatory substance.
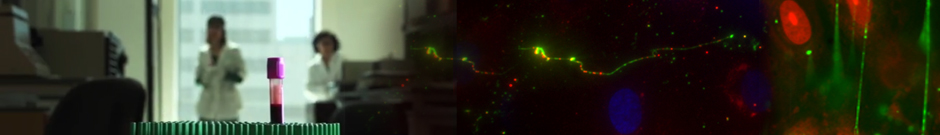
 Left: blood platelets (purple) adhere to von Willebrand factor (VWF) multimeric strings that are secreted by, and anchored to, vascular endothelial cells. Image by N. Turner of the Moake Laboratory in Hematological Research
Left: blood platelets (purple) adhere to von Willebrand factor (VWF) multimeric strings that are secreted by, and anchored to, vascular endothelial cells. Image by N. Turner of the Moake Laboratory in Hematological Research