Research
For over 25 years, the Moake Laboratory in Hemostasis-Thrombosis Research has investigated the basic mechanisms of hemostasis-thrombosis under flowing conditions, and the pathophysiology of thrombotic microangiopathies. More specifically this has included:
- Shear stress-induced, von Willebrand factor (VWF)-mediated platelet clumping;
- Pathophysiology and therapy of thrombotic thrombocytopenic purpura (TTP), and hemolytic-uremic syndromes HUS), and other types of thrombotic microangiopathies; and
- Molecular interactions between VWF and complement.
The laboratory team was first to discover that:
- Unusually large VWF (ULVWF) multimers secreted from endothelial cells are not processed properly, and cause systemic platelet aggregation, in TTP (1982; publication # 1 on publications page);
- High shear stress-induced platelet aggregation requires large VWF multimers, (1986; publication # 3 on publications page);
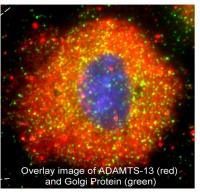
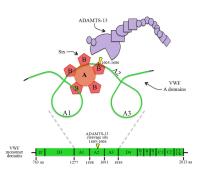
- Inhibition of the VWF-cleaving protease, ADAMTS-13, by Shiga-like toxin from Escherichia coli contributes to ULVWF-mediated thrombosis in the microcirculation of the kidney in the common diarrhea-associated type of HUS (2005 and 2013; publications # 11 and 16 on publications page);
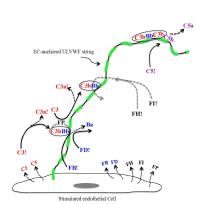
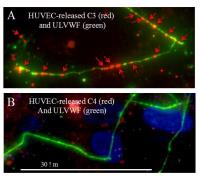
- The alternative complement pathway is initiated and amplified by complement component C3 binding to ULVWF multimers secreted by, and anchored to, human endothelial cells (2013; publication # 19 on publications page), providing a molecular linkage between hemostasis-thrombosis and inflammation; and
- Factor H, a regulatory protein in the alternative complement pathway, also has reductase activity for soluble VWF multimers (2013; publication # 17 on publications page).
Current laboratory emphasis
New research in the Moake laboratory looks at molecular interactions between the earliest events in hemostasis-thrombosis (VWF) and inflammation (alternative complement pathway).